The educational content in this post, elaborated in collaboration with Bromatech, was independently developed and approved by the GMFH publishing team and editorial board.
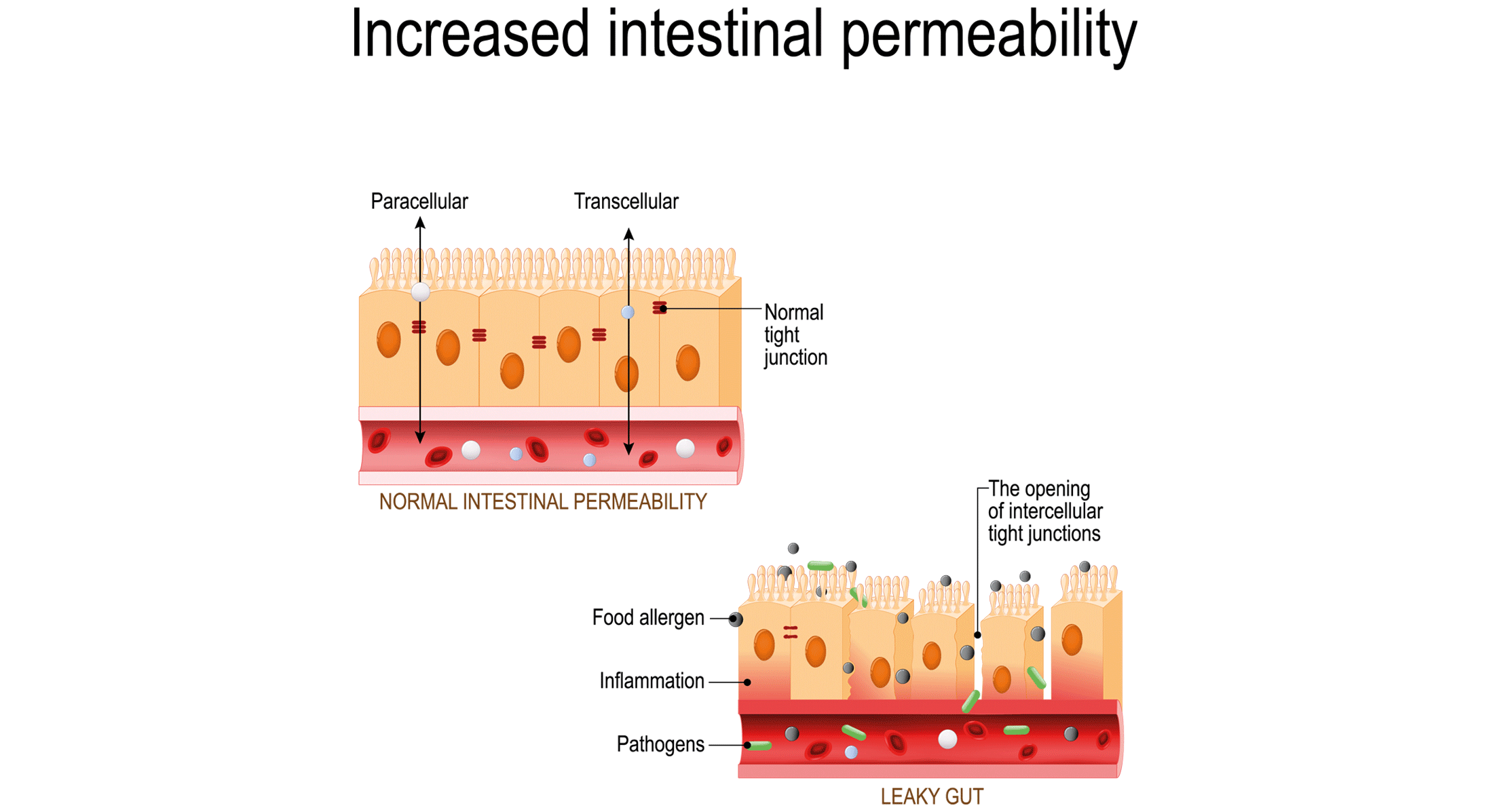
What does increased intestinal permeability or ‘leaky gut’ mean?
The term ‘leaky gut’ is the simplistic concept used to reflect increased intestinal permeability due to a dysfunction in any of the gut barrier’s components. Indeed, a leaky gut goes beyond an altered epithelial cell layer and can also affect the mucus layer and inner layer, including immune cells that are important components of the gut barrier.
Professor Giovanna Traina from the University of Perugia explained via email to GMFH editors that “a healthy intestinal barrier is essential for the maintenance of gastrointestinal health, but also for the systemic health of the host, as it is able to preserve the potential translocation of bacteria, food allergens, xenobiotics and inflammatory mediators in the systemic circulation, that may compromise the functionality of other organs.”
Such a defect in the gut barrier may facilitate dietary and microbial antigen influx, leading to the chronic inflammation involved in the onset of modern diseases. Examples of diseases and disorders that have been linked to increased intestinal permeability include gastrointestinal disorders, enteric infections, obesity and metabolic syndrome, liver diseases, pancreatitis, autoimmune diseases and neuropsychiatric diseases. An altered intestinal barrier function can also be a result of host physiological factors (e.g., bile acids) and environmental factors (e.g., dietary components) and is not necessarily deleterious, leading to a disease phenotype.
How can you diagnose a leaky gut in your clinical practice?
Several techniques involving biopsy and urine samples have been used to assess barrier function and intestinal permeability. While none of the available tests is without its shortcomings, the lactulose/mannitol test is the most widely employed and focuses only on the permeability function.
“The lactulose/mannitol ratio index is highly increased in intestinal inflammation and in pathologies characterized by an increased intestinal permeability, such as Crohn’s disease,” acknowledges Prof. Traina. When interpreting the test’s results, it is important to keep in mind that both lactulose and mannitol are metabolized by the colonic microbiota, thus cannot be used in ulcerative colitis or irritable bowel syndrome, which mostly affect the colon.
Beyond the lactulose/mannitol test, Prof. Traina stated that the levels of fecal calprotectin, a protein released by the activation of neutrophils, can be measured as a marker of intestinal permeability induced by intestinal inflammation. On the apical area in close contact with the intestinal lumen, enterocytes are linked by tight junctions and impaired intestinal permeability may result from an increase in zonula occludens proteins. However, “data in literature are not yet entirely in favor of its use, as demonstrated by recent work in which researchers advise to be careful in considering the measurement of serum zonulin as a marker of the integrity of the intestinal barrier,” states Prof. Traina.
Other techniques measure barrier function in a broader sense, for instance bacterial translocation of lipopolysaccharide or epithelial cell damage with assays measuring fatty acid binding protein or citrulline. The measure of bacterial metabolites such as butyrate in serum or feces is another approach, due to its role in enhancing colonic barrier function. For the most part, the techniques have been applied in an experimental setting and their application in clinical practice remains to be seen.
Is there a role for dietary interventions in reducing the leakiness of intestinal permeability?
Prof. Traina explained that various factors can lead to perturbations in the structural dynamics of microbiota and to changes in the functional characteristics of the intestinal barrier. They are environmental factors, diet, genetic defects, stress and drugs.
Within that group, diet is one of the most important in its effect on barrier function. A diet high in saturated fats, fructose, emulsifiers and alcohol ingestion, along with vitamin A deficiency and changes in diet or the microbiota that lower butyrate levels can impair barrier function and increase permeability. Prebiotic fibers (especially derived short-chain fatty acids), probiotics, polyphenols, glutamine, methionine, vitamin D and zinc can, in contrast, enhance barrier integrity. All such nutrients and dietary interventions can be ingested through food staples and supplements.
Probiotic bacteria and yeasts are being increasingly studied due to their positive effects on overall gut integrity. That is the case of the multi-strain probiotic formulation consisting of Lactobacillus rhamnosus, Bifidobacterium lactis and B. longum, which has been shown in two recent in vitro studies led by Prof. Traina (here; here) to preserve the integrity and functioning of the intestinal barrier from the damage caused by the lipopolysaccharide inflammatory stimulus. The findings support the notion that specific probiotics are a plausible approach to enhancing epithelial barrier function in a specific manner, but that preliminary research should be confirmed in well-designed randomized clinical trials.
It is important to understand that while improving gut barrier function is increasingly used in the clinical setting as a therapeutic goal, the evidence is not yet there to support the efficacy of a single intervention in curing a disease by restoring or improving barrier function.
Take-home messages
- A reduced barrier function with increased permeability or ‘leaky gut’ has been associated with intestinal and extraintestinal diseases.
- A wide range of techniques are used for assessing barrier integrity and function but each one has its own limitations that should be kept in mind when interpreting their results.
- Probiotics are among the key dietary interventions being investigated due to their potential for producing positive effects on gut integrity and thus improving intestinal permeability, which is impaired in some conditions.
- Reversing an altered intestinal barrier function may be necessary but may not be sufficient to reverse disease pathogenesis, as other factors such as immune response play a role in perpetuating the disease.
References:
Camilleri M, Vella A. What to do about the leaky gut. Gut. 2022; 71(2):424-435. doi: 10.1136/gutjnl-2021-325428.
Quigley EMM. Leaky gut – concept or clinical entity? Curr Opin Gastroenterol. 2016; 32(2):74-79. doi: 10.1097/MOG.0000000000000243.
Wyatt J, Vogelsang H, Hübl W, et al. Intestinal permeability and the prediction of relapse in Crohn’s disease. Lancet. 1993; 341(8858):1437-1439. doi: 10.1016/0140-6736(93)90882-h.
Ikhtaire S, Sharif Shajib M, Reinisch W, et al. Fecal calprotectin: its scope and utility in the management of inflammatory bowel disease. J Gastroenterol. 2016; 51(5):434-446. doi: 10.1007/s00535-016-1182-4.
Ajamian M, Steer D, Rosella G, et al. Serum zonulin as a marker of intestinal mucosal barrier function: may not be what it seems. PLOS ONE. 2019; 14(1):e0210728.
Bron PA, Kleerebezem M, Brummer RJ, et al. Can probiotics modulate human disease by impacting intestinal barrier function? Br J Nutr. 2017; 117(1):93-107. doi: 10.1017/S0007114516004037.
Sichetti M, De Marco S, Pagiotti R, et al. Anti-inflammatory effect of multistrain probiotic formulation (L. rhamnosus, B. lactis, and B. longum). Nutrition. 2018; 53:95-102. doi: 10.1016/j.nut.2018.02.005.
de Vito R, Conte C, Traina G. A multi-strain probiotic formulation improves intestinal barrier function by the modulation of tight and adherent junction proteins. Cells. 2022; 11(16):2617. doi: 10.3390/cells11162617.