Gut-Microbiota-Brain Axis: Interactions between Microbiome, Central Nervous System and Enteric Nervous System
Microbiome & Microbial Therapy (MMT)-related sessions kicked off with emerging evidence on mechanisms by which bacterial metabolites influence neuronal signaling and describing sex specific differences in microbiome and effects on visceral pain.
Jing Lu from the University of Chicago presented findings from her work on maternal probiotics supplementation with Limisilobactobacillus reuteri to improve neurodevelopment in the offspring.
Maternal immune activation (MIA) derived from late gestational infection such as seen in chorioamnionitis poses a significantly increased risk for neurodevelopmental deficits in the offspring. Lu et al modeled MIA in mice by exposure to lipopolysaccharide (LPS) at gestational week 16; supplemented them with L. reuteri during lactation and evaluated the alterations in microbiome composition, metabolic profile and blood-brain barrier development and function of the offspring. This experimental design allowed them to show that maternal supplementation with L. reuteri starting at birth rescues the LPS-driven blood-brain barrier (BBB) underdevelopment and dysfunction-associated cognitive function. That was mediated by alterations in β-diversity of the microbial community and metabolic responses in the offspring at week 2 after birth but not week 12.
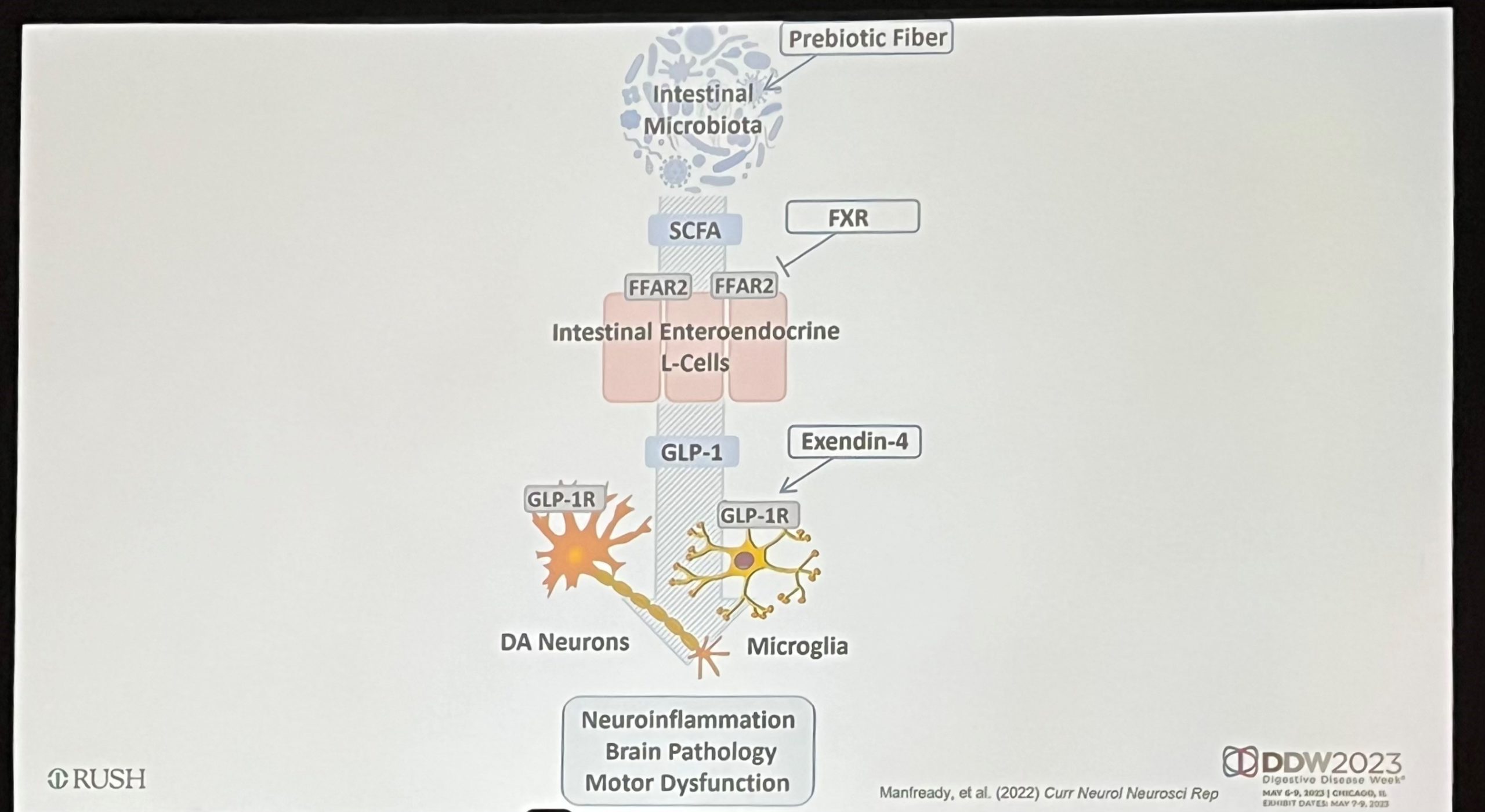
In the same session, Richard Manfready from the Rush Center for Integrated Microbiome and Chronobiology Research in Chicago, covered findings from the Ali Keshavarzian Lab on gut microbiota in Parkinson’s Disease (PD).
This combinatory human and in vitro work explored three aspects of microbial composition, namely metabolic capacity, enteroendocrine L-cells activation and glucagon-like-peptide-1 mediated barrier (dys)function. Manfready et al concluded that PD-related microbial dysbiosis is associated with decreased abundance of: (1) short chain fatty acids (SCFA)-producing bacteria, (2) serum and stool SCFAs concentration, (3) GLP-1 secretion, (4) intestinal barrier integrity, and (5) fecal calprotectin. Overall, these alterations mediated by PD can potentially be mitigated by prebiotic supplementation.
What’s new in the management of disorders of gut-brain interaction?
Some presentations focused on recent advances in the dietary and pharmacological management of disorders of gut-brain interaction, mainly IBS. It was emphasized that a multidisciplinary care is needed in the management of gastrointestinal disorders and gastrointestinal registered dietitians can be involved in training gastrointestinal fellows. Heidi Staudacher presented a poster of a randomised clinical trial showing that a 6-week Mediterranean diet is a feasible and potentially therapeutic dietary intervention for reducing gut and psychological symptom burden in IBS, highlighting that restrictive diets such as the low FODMAP diet are not always the unique solution for these patients. It is also important to screen for avoidant restrictive food intake disorders or eating disorders before following a restrictive diet and in that regard registered dietitians are instrumental.
William D. Chey covered available treatment options for abdominal bloating that is an uncomfortable symptom for most patients with digestive issues. Beyond diet, belly breathing is a useful intervention that can assist for improving gastric accommodation, bloating and abdominophrenic dyssynergia. William D. Chey and Lin Chang also presented a clinical practice guideline collaboration with the American Gastroenterological Association and the American College of Gastroenterology on pharmacotherapy for chronic idiopathic constipation available here.
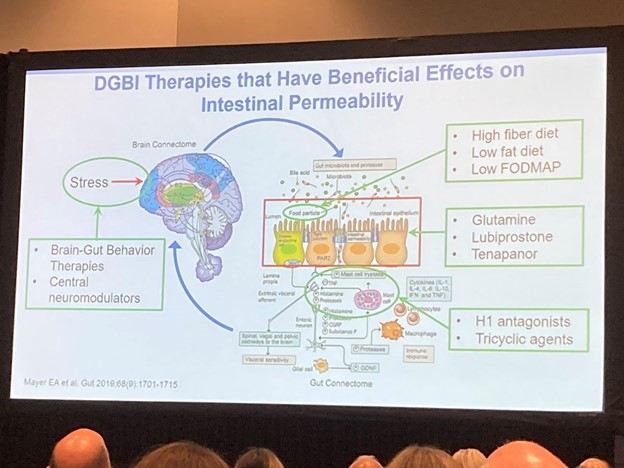
Intestinal permeability-related disorders were also addressed in a session. However, the popular term “leaky gut syndrome” should be avoided as is a vague term used by some experts to sell ineffective supplements and restrictive diets. While a leaky gut or increased intestinal permeability exists, the science does not allow generalizing that it is the cause of a multitude of ailments. An altered barrier function may lead to an uncontrolled exchange of fluids and solutes between the intestinal lumen and surrounding tissues. But while intestinal permeability can be measured in clinical practice, Tim Vanuytsel acknowledged that commonly used blood markers like LPS and zonulin are not validated measures.
While the small bowel microbiome has been largely overlooked, Mark Pimentel and colleagues showed the first shotgun sequencing of the small intestinal bacterial overgrowth (SIBO) revealing that SIBO is linked with 3 species of bacteria including Escherichia coli, Klebsiella pneumoniae and Klebsiella aerogenes that are associated with gastrointestinal symptoms. They also found link between increased fungi in duodenum and severe upper respiratory symptoms and abdominal pain. Pimentel’s team also presented new data into the impact of artificial sweeteners on the small bowel microbiome which can explain why patients with bloating and altered bowel habits tolerate worse sugar alcohols and sugar-derived sweeteners like sucralose than amino acid-derived sweeteners like aspartame (for further reading on advances on SIBO dysbiosis and gastrointestinal symptoms see here).
References:
Lu J., Fan X., Lu L., Yu Y., et al. Limosilactobacillus reuteri normalizes blood-brain barrier dysfunction and neurodevelopment deficits associated with prenatal exposure to lipopolysaccharide Gut microbes 2023 doi: 10.1080/19490976.2023.2178800.
Manfready R.A., Forsyth C.B., Voigt R.M., Hall D.A. et al. Gut-Brain Communication in Parkinson’s Disease: Enteroendocrine Regulation by GLP-1 Curr Neurol Neurosci Rep 2022 doi: 10.1007/ s11910-022-01196-5
Chang L, Chey WD, Imdad A, et al. American Gastroenterological Association-American College of Gastroenterology Clinical Practice Guideline: pharmacological management of chronic idiopathic constipation. Gastroenterology. 2023; 164(7):1086-1106. doi: 10.1053/j.gastro.2023.03.214.
Fairlie T, Shah A, Talley NJ, et al. Overlap of disorders of gut-brain interaction: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2023; 8(7):646-659. doi: 10.1016/S2468-1253(23)00102-4.
Leite G, Rezaie A, Mathur R, et al. Defining small intestinal bacterial overgrowth by culture and high throughput sequencing. Clin Gastroenterol Hepatol. 2023. doi: 10.1016/j.cgh.2023.06.001 [online ahead of print]


