Babies are born with immature immue systems. Until now, it was believed that the birth process was the first opportunity for microorganisms from the mother to colonize the baby’s gut and, thus, to shape the immune system.
Now, a team formed by German and Swiss scientists have discovered that this interaction with the baby’s immune system starts much earlier than previously thought: during pregnancy.
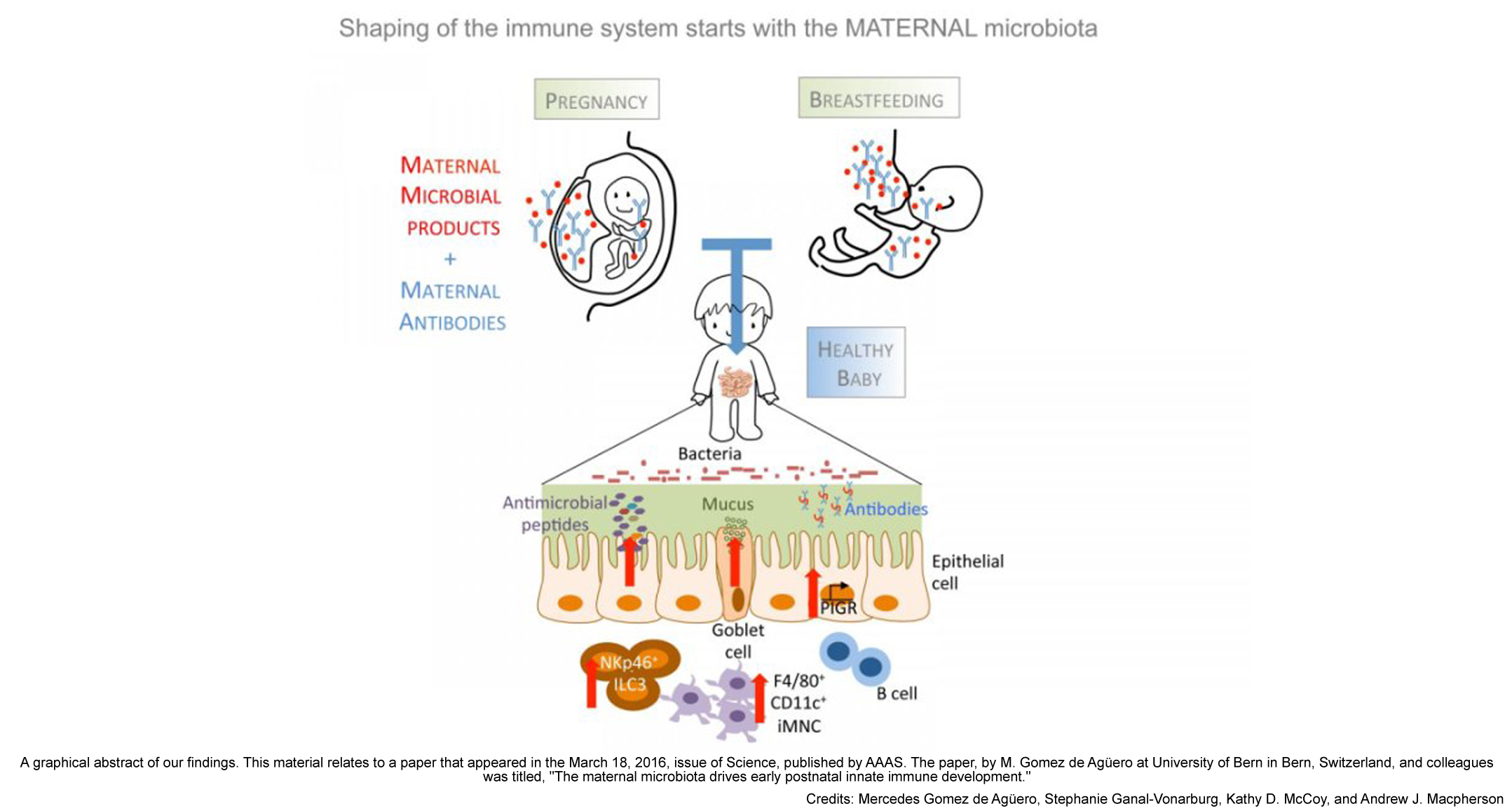
According to their findings, published in Science magazine, some molecules produced by the gut microbiota and/or non-living bacterial fragments are transferred from the mother to the baby through the placenta or, later, through antibodies present in breast milk. These molecules and fragments may stimulate immune cells in the newborn to prepare it for coping with microorganisms living in its own gut.
To demonstrate this in an experiment with rodents, researchers raised germ-free pregnant mice and then colonised them temporarily with E. coli engineered to lessen over time and allow the mothers to be germ-free at the moment of labour.
Much to their surprise, the researchers observed that this colonization of the mother with E. coli affected the immune system of the offspring: after birth, those pups born to colonised mothers had increased numbers of certain immune cells (such as lymphoid and mononuclear cells) in their guts compared to pups from completely microbe-free mothers. And those immune cells persisted even after weaning.
“We observed that during pregnancy, small lifeless fragments from bacteria in the mother’s gut passed through the placenta and [arrived in] the foetus to start shaping the immune system of the pup”, points out Mercedes Gómez de Agüero, researcher at University of Bern (Switzerland), and first author of the paper, in an interview with Gut Microbiota for Health.
“We did not find [live] bacteria, [either] in the placenta [or in] the foetus. Our work [shows] the fact that the colonisation with live bacteria starts after birth”, says Gómez de Agüero.
In fact, those lifeless molecules Gómez de Agüero refers to prepared the offspring for bacterial colonisation after birth. She saw an expansion of cells in the immune system of the baby and an activation of gene expression involved in the formation of the intestinal barrier, responsible for the containment of the microbiota within the gut.
Indeed, the groups of animals presented different patterns of gene expression in their intestines. As compared to pups born to germ-free mothers, pups born to microbe-exposed mothers had a greater expression of numerous genes, such as those influencing cell division and differentiation, metabolism and, of course, immune function.
But why does this process of microbial influence start during gestation? The authors suggest in their paper that this microbial influence on offspring during pregnancy may have a key role in preparing the immune system to be exposed to the large variety of microbes that will inhabit the gut throughout life.
“The newborn is then able to control the bacteria of his or her own intestine and the immune system can respond in an attenuated and proportioned way to the presence of bacterial molecules in the organs”, Gómez de Agüero explains to Gut Microbiota for Health in an email. “The fact that the immune system of the newborn is prepared during pregnancy is what allows him to develop this suitable response to control the bacterial colonisation without developing an exaggerated response or being infected”.
In fact, researchers observed that the immune systems of pups born to mothers colonised during pregnancy also seemed to protect against inflammation and bacterial infection. “Being aware of when the immune system of the baby starts preparing could shed light on how to improve that preparation in order to prevent related children’s diseases”, says Gómez de Agüero.
The experiment showed transfer of ‘information’ to the immune system is also carried out though maternal milk, as mice born to germ-free mothers and nursed by colonised moms also showed an increase in certain types of immune cells.
Although these findings are based on an animal model, they may provide new insights into the early development of the immune system in humans. The work shows one of the mechanisms by which the maternal microbiota during pregnancy may exert its powerful effect on the future development of the infant.
References:
Mercedes Gómez De Agüero, Stephanie C. Ganal-Vonarburg, Tobias Fuhrer, et al. The maternal microbiota drives early postnatal innate immune development. Science, 2016DOI: 1126/science.aad2571