Recently, there has been a huge change in the perception of microbial diseases and a shift in the understanding of how to treat them. It is now recognized that there are few disorders that can be attributed to the presence or absence of a single organism. Rather, the structure of the entire community of microbes constituting the human microbiota often determines the disease state.
Most mucosal surfaces of the human body contain an extremely complex ecosystem of microbial organisms. These resident microbes exist in a delicate balance with each other and the human host that is dictated by the specific local environmental factors of each host body site. In health, the many different species that make up the microbiota exist in homeostasis with the host immune system. A dysbiosis of the microbiota shifts the community, which may lead to disease.
Since the advent of antibiotics, the treatment of microbial diseases has focused on non-specifically eliminating entire groups of bacteria. The overuse of broad-spectrum antibiotics has led to the many adverse effects we are currently dealing with in the so-called “post-antibiotic era”, including antibiotic resistance and antibiotic-induced diseases, such as C. difficile infections. New treatment strategies for microbial diseases need to reflect this increased understanding of the microbial ecology of the human body. One strategy under investigation is the development of targeted antibiotics which can selectively kill specific pathogens within the microbiome.
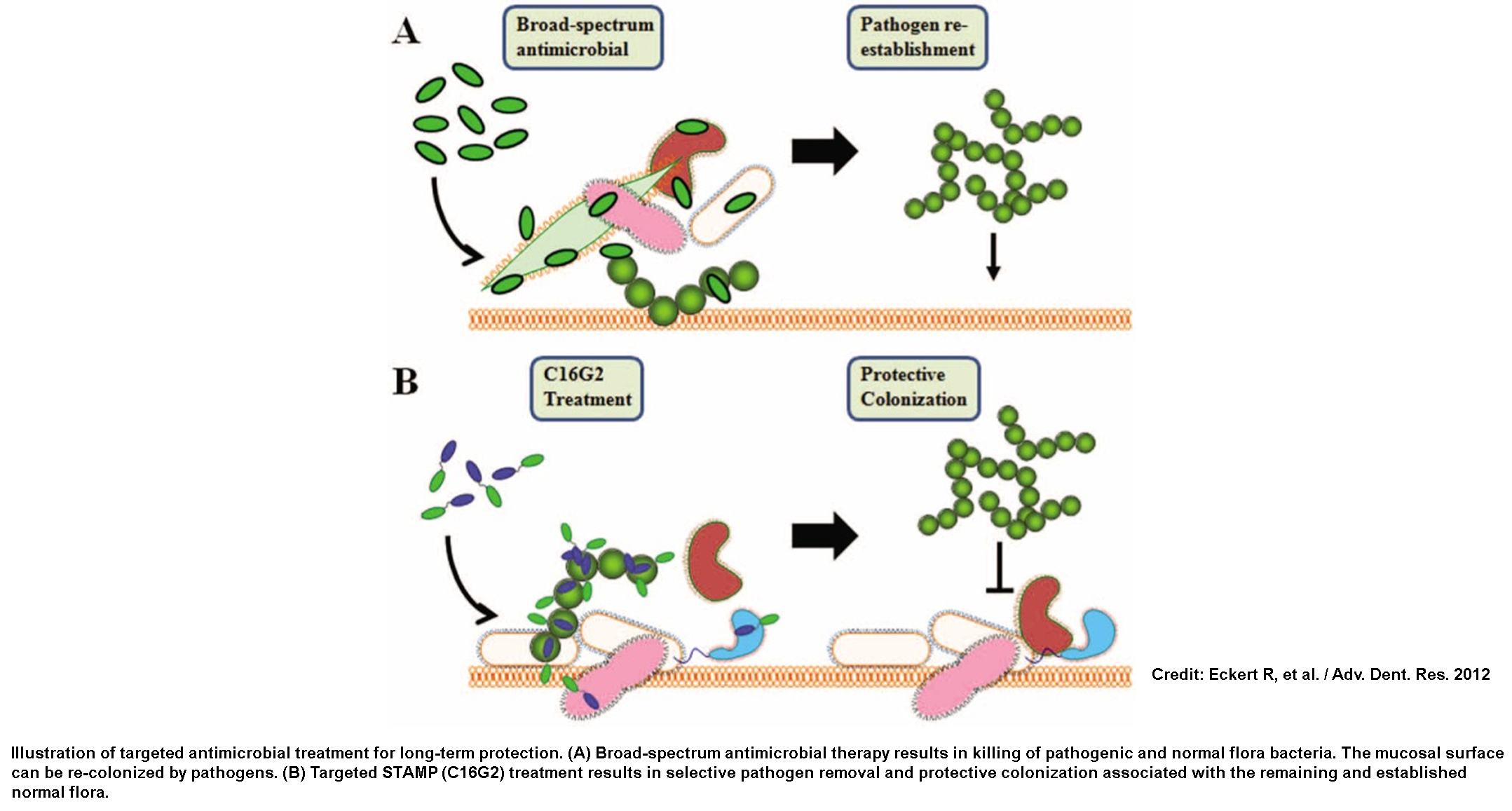
The microbiome of the human oral cavity consists of ~700 different species [1] in which a dysbiosis leads to various diseases such as dental caries and periodontal disease. Oral microbes exist in a highly structured society that is regulated by complex signaling mechanisms. The species of the oral microbial community are physically as well as physiologically connected, with metabolically complementary organisms relying on each other and the rest of the community for nutrients needed for growth. In this way, the removal of a single species can lead to a change in the entire community structure [2]. S. mutans is one of the organisms that contributes to dental caries [3] due to its ability to generate acid from dietary sugar as well as thrive in acidic conditions. Current treatments for caries include surgical (drilling and filling) or fluoride-based therapies, but neither effectively eliminates the underlying microbial cause.
In theory, broad-spectrum antibiotics would prevent caries by eliminating most oral bacteria. However, following cessation of treatment, S. mutans will easily return due to the removal of competition from resident commensal species. Studies show [2,4] that the targeting killing of S. mutans leads to a re-structuring of the entire oral microbial community. The void created by the removal of S. mutans is quickly filled by other, health-associated Streptococcus species, and prevents the re-introduction of S. mutans through protective colonization. The removal of S. mutans allows a protective community to form, one that is no longer friendly to this pathogen.
The idea of precision-guided antimicrobials is currently being applied to other disease states and body sites, including Clostridium difficile infections [5], a debilitating disease triggered by the use of broad-spectrum antibiotics. The ability to selectively remove a single organism from a complex community has the potential to be a game-changer for antibiotic development as well as microbiome research.
References:
[1] Dewhirst FE, Chen T, Izard J, et al. The Human Oral Microbiome. J Bacteriol. 2010; 102:5002-5007.
[2] Guo L, McLean JS, Yang Y, et al. Precision-Guided Antimicrobial Peptide as a Targeted Modulator of Human Microbial Ecology. Proc. Natl. Acad. Sci. U.S.A. 2015; 112:7569-7574.
[3] Marsh PD Dental plaque as a biofilm and a microbial community – implications for health and disease. BMC Oral Health. 2006; 6(Suppl 1):S14.
[4] Eckert R, et al. Targeted antimicrobial treatment to re-establish a healthy microbial flora for long-term protection. Adv. Dent. Res. 2012; 24:94–97.
[5] Gebhart D, Lok S, Clare S, et al. A Modified R-Type Bacteriocin Specifically Targeting Clostridium difficile Prevents Colonization of Mice without Affecting Gut Microbiota Diversity. mBio. 2015; 6(2).