Previous research has shown that the gut microbiota may have a role in the pathogenesis of immune-related diseases involving chronic inflammation, such as rheumatoid arthritis, Crohn’s disease, multiple sclerosis, and others.
A recent review, led by Dr. Hai Lu from the Department of Orthopedic Surgery at The Third Affiliated Hospital of Southern Medical University in Guangzhou (China), discusses the existing evidence for the role of gut microbiota in ankylosing spondylitis.
Ankylosing spondylitis (AS) is a chronic inflammatory disease that mainly affects the sacroiliac joints and spine. Although in its pathogenesis appears to involve both genetic and environmental factors, the exact causes that determine individual’s susceptibility to AS remain unknown.
When considering genetic background, human leukocyte antigen B27 (HLA-B27) is a major risk factor for AS, present in up to 95% of AS patients. However, genetics alone does not explain the cause of the disease and evidence of AS-microbiome interactions in humans comes from the observation that AS patients have higher levels of anti-Pophyromonas gingivalis antibody titers (P. gingivalis being a periodontal pathogen). Therefore, it seems that periodontal pathogens could contribute to the development of AS in genetically susceptible individuals. The authors also mentioned that other bacteria that may play a role in AS pathogenesis are Klebsiella pneumoniae, K. aerogenes and Bacteroides vulgatus.
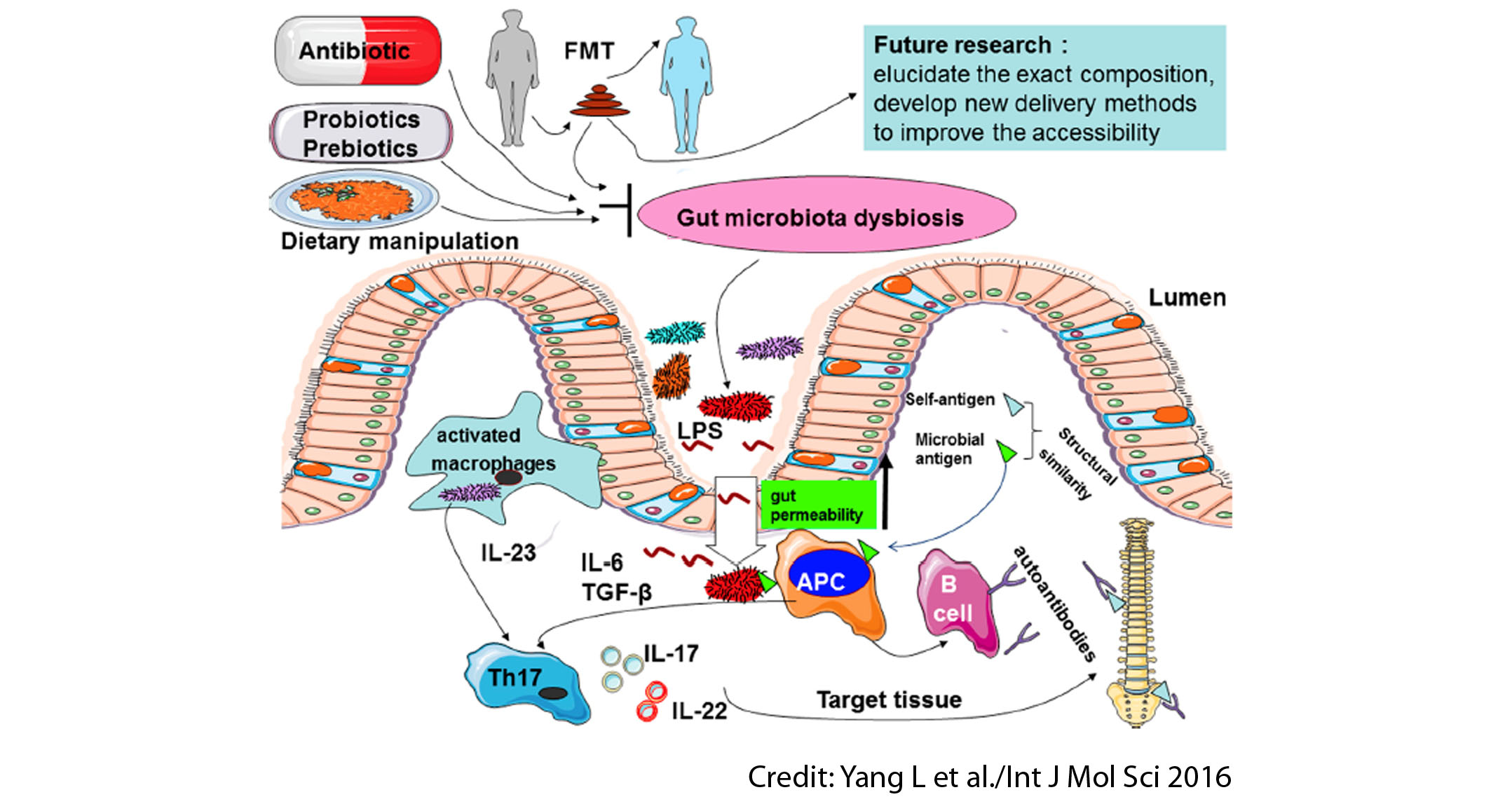
Several kinds of evidence suggest that gut microbiota may be a possible contributor to AS development. The mechanisms that explain it include subclinical gut inflammation, alterations of intestinal permeability, microbial translocation, stimulation of immune responses, and molecular mimicry. There is a protective role of breastfeeding. The overall picture is that AS may be triggered by a disturbed gut environment and gut microbiota dysbiosis, which may cause inflammation and exacerbate the disease.
The review wraps up with an overall discussion about the role of microbiota-based therapeutics as potential AS treatments. Potential therapeutic strategies aiming to reduce the gut microbiota dysbiosis related to AS might include:
- Antibiotics: sulfasalazine and moxifloxacin have been shown to reduce bacterial loads in the bowel of AS patients and reduce disease exacerbations.
- Probiotics and prebiotics: the probiotics Lactobacillus casei and Lactobacillus rhamnosus GG and the prebiotic inulin-oligofructose alleviated tissue damage and decreased inflammatory status in rats.
- Dietary manipulation: a low-starch diet is reported to reduce inflammation and lead to clinical remission when adopted alongside current medical treatment in small samples of active AS patients.
In conclusion, although mounting evidence suggests a possible role of the gut microbiota in AS pathogenesis, further studies are needed in order to confirm the underlying mechanisms. Whether antibiotics, probiotics, prebiotics, and low-starch diets can be used as strategies to reduce gut microbiota dysbiosis in AS remains ripe for exploration. Faecal microbial transplantation has not been evaluated at all so far in AS.