Ever wonder why the diet your doctor prescribed, which you followed for several months, failed to work despite all your efforts? Or why although you eat healthily you can’t lose a measly pound while your gym mates are all slimming down?
Researchers from the Weizmann Institute of Science in Israel have now discovered and proven for the first time the reason why nutritional interventions do not have the same effect on us even if we all eat the same food. And, even worse, how supposedly healthy meals may even contribute to exacerbating weight problems in some individuals.
In a study of almost 1000 participants, the results of which were published in November in Cell, scientists found that each person metabolises food very differently and that our gut microbiota has some say in this huge variability between individuals.
“These findings have an enormous impact on the way we have understood nutrition until now, moreover taking into account World Health Organization (WHO) alerts regarding obesity and diabetes, two major metabolic conditions that together affect almost half the world’s population, with diet being the main medical intervention for treating these conditions,” explained researcher and co-author of the study Eran Elinav from the Weizmann Institute of Science’s Department of Immunology during a press conference.
Changes in our diet over the past four decades are thought to have contributed to the prevalence of these metabolic conditions. “That is what lead us four years ago to start thinking about taking a scientific approach to the problem of nutrition,” added co-author and researcher Eran Segal.
Eight hundred volunteers from Israel were recruited, some of them pre-diabetics, and they were monitored over a two-week period. Researchers collected health data about them through questionnaires, body measurements and blood tests. They also connected participants to a small device that monitored their blood glucose level (a known risk factor for metabolic diseases) every five minutes. In addition, volunteers were asked to report about their lifestyle and food intake through an app specifically designed for the experiment.
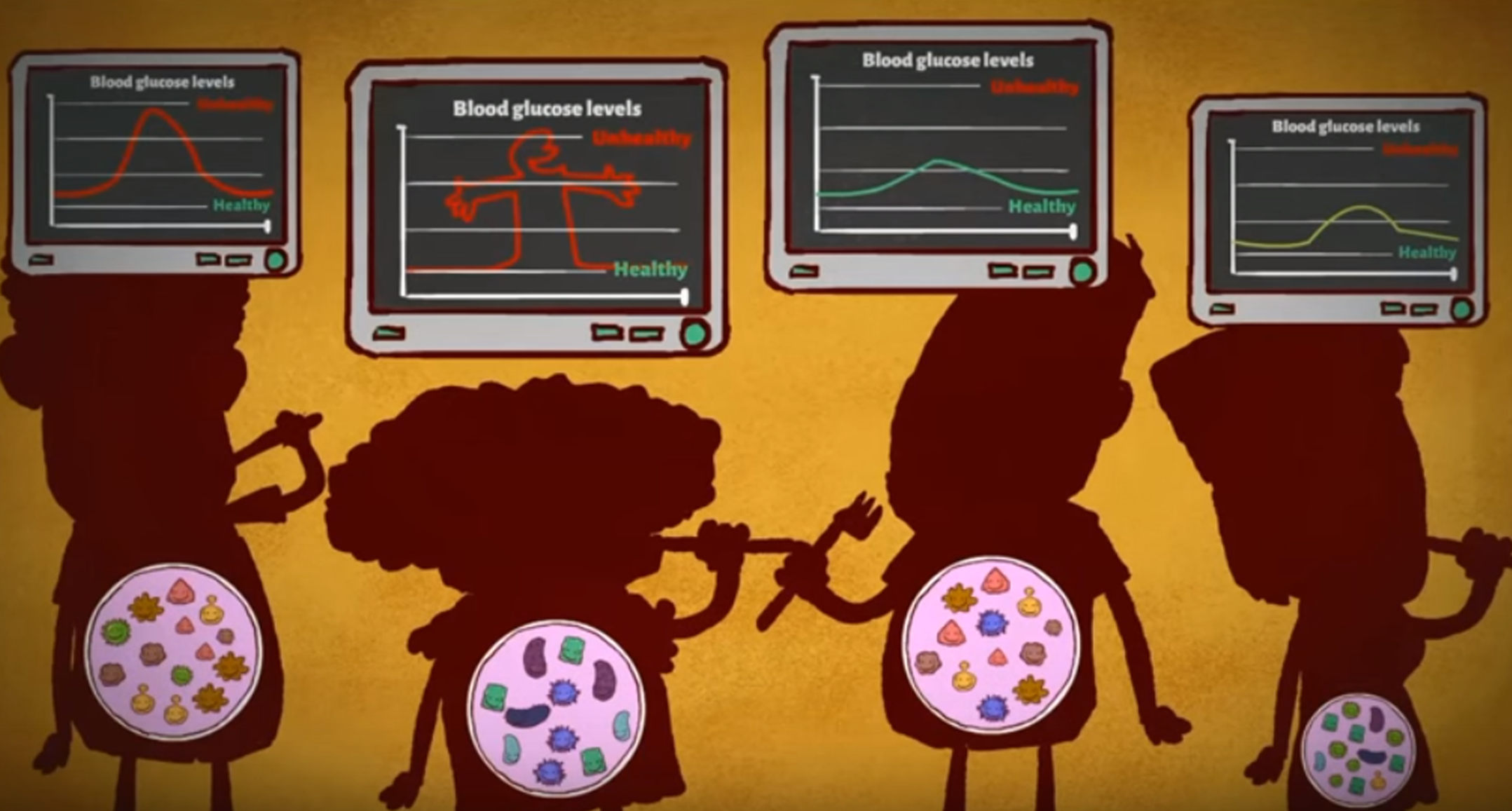
Researchers focused on post-meal responses and on how blood sugar levels changed in the two hours following a meal. The data they gathered revealed that individuals have vastly different responses to the same food. For instance, some people’s glycaemic responses spiked after eating a tomato, whereas other individuals did not experience the same effect. “Our first surprise was to discover on a very large scale the enormous variability we saw in people’s responses to even identical meals. There are profound differences between individuals. In some cases, individuals even have opposite responses and this is a big hole in the literature,” Segal pointed out.
Using all the data gathered, they also created an algorithm with which they were able to predict the blood sugar level response of the 800 participants. In addition, to check the efficiency of the algorithm, they recruited 100 more people and were also able to predict their blood glucose levels after eating a particular food.
In order to understand why such vast differences exist between people and knowing that several previous studies had linked the gut microbiota to obesity and diabetes, the researchers decided to collect and analyse stool samples from each participant. As suspected, they found that specific gut microbe species correlated with post-meal blood sugar reactions.
They also carried out a new experiment, for which they recruited a small cohort of 26 additional participants, taking stool samples from each one. Then, based on the algorithm they had designed, they prescribed a personalised diet for each of them, some of which were intentionally ‘healthy’, while others were not.
Scientists measured the response of blood sugar levels after each meal for a week and then collected new stool samples. They observed that the participants who had a low blood sugar level response post-meal had also experimented changes in their gut microbiota composition. For instance, higher amounts of the bacteria associated with the improvement of glucose tolerance (Actinobacteria) were found in the stools, whereas the bacteria linked to diabetes (Proteobacteria and Enterobacteriaceae) were less abundant.
“We showed in our study that the gut microbiota is a major component responsible for people’s responses to food,” said Elinav. “We also suggest that we may be able to start harnessing the microbiota’s function to improve people’s health. In the future, as our findings suggest, we might be able to modify microbiota composition through diet and personalised probiotics and maybe this would help us improve people’s propensity to develop common diseases.”
Interested in learning more about this study? Have a look at its video abstract!