The identification of an isolate at the strain level has been considered an essential requirement for any microbe that is intended to be commercialized as a probiotic. Strain-level identity is essential for both safety and efficacy evaluations. It enables traceability in laboratory tests, clinical trials and throughout the production and commercialization process. Although the health benefits of probiotics have been considered to be strain-specific for decades, scientists are now recognizing commonalities among members of taxonomic groups, which may be at the root of some beneficial effects.
For instance, the International Scientific Association for Probiotics and Prebiotics’ consensus document on the scope and use of the term ‘probiotic’ pointed out that some effects and mechanisms that support them are broadly distributed among species within a genus (colonization resistance and short-chain fatty acids production etc.), others are frequent among different strains of the same species (vitamin synthesis, gut barrier reinforcement, etc.) and, finally, others appear to be less widely distributed among probiotic strains (neurological effects or immunological effects, etc.).
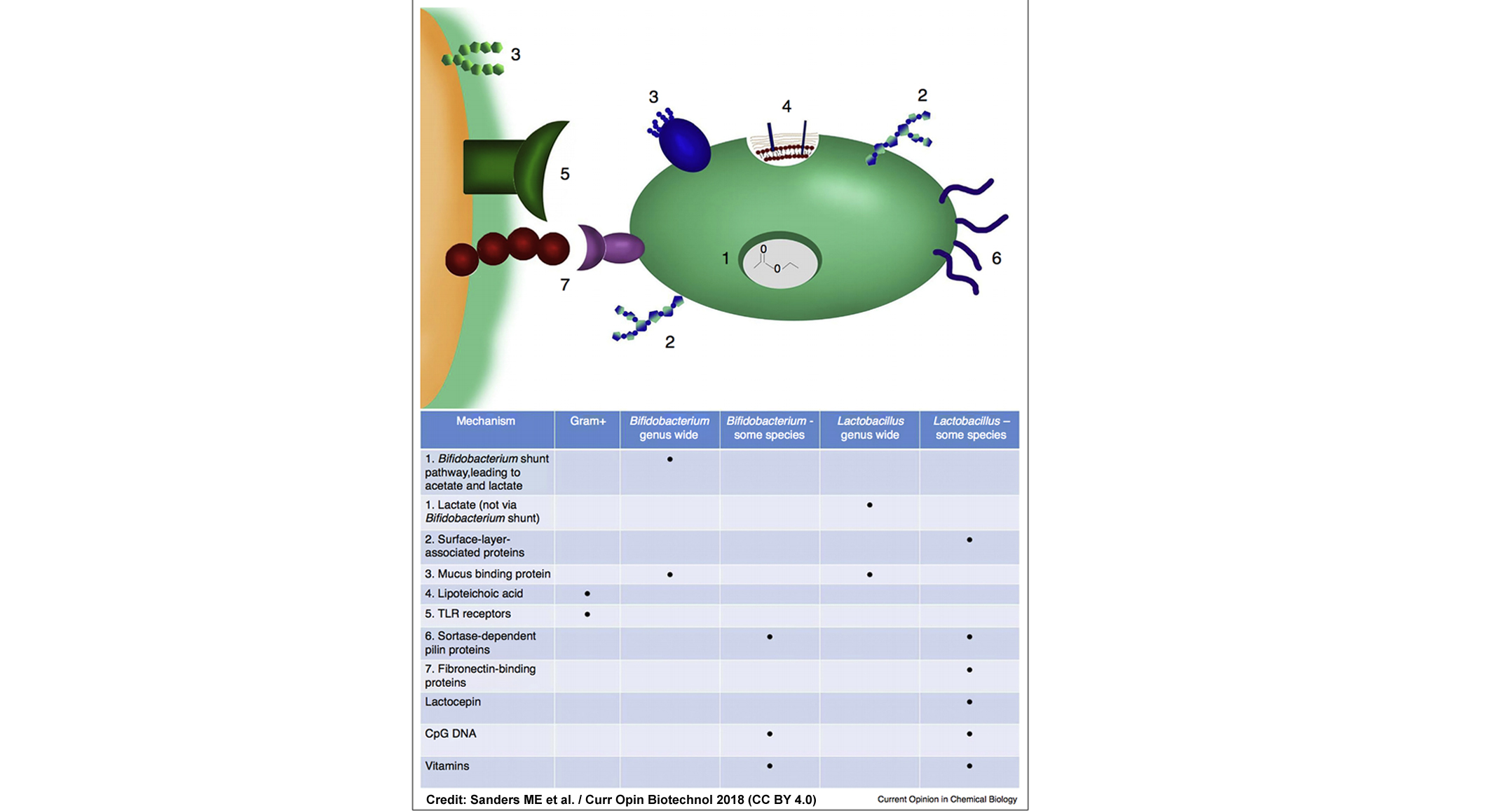
Mary Ellen Sanders and colleagues recently published a review of the implications of the fact that probiotic benefits may derive from shared mechanisms within taxonomic groups that go beyond strain-specificity.
Below, Sanders answers some questions from GMFH editors about some of the recent paper’s relevant findings.
Why has strain-specificity of probiotic effects been a cornerstone principle of probiotic science for decades?
Probiotics are clearly different at the strain level; this has been aptly demonstrated in many laboratory and animal assessments. So the correct approach to the field is to assume that, in the absence of evidence to the contrary, different strains were thought to likely confer different health effects.
What does your recent review add to what is known regarding general probiotic claims?
The concept behind this recent review was first suggested in a commentary by Prof. Hill and me in 2013 titled Rethinking “Probiotics”. The backdrop is that although probiotic strains differ, evidence is accruing that common mechanisms exist among some groups of bacteria that may translate into their ability to confer similar health benefits. This new review outlines some shared mechanisms—production of short chain fatty acids and cell surface architecture—that may drive clinical benefits delivered by groups of bacteria.
Are there basic benefits that can be attributed to a probiotics’ general category? If so, is probiotic classification at genus or species level sufficient?
The Hill et al (2014) paper concluded that sufficient evidence exists that digestive benefits could be expected from strains of certain well-studied probiotic species. In other words, using the term ‘probiotic’ to describe species such as Lactobacillus rhamnosus or Bifidobacterium lactis is scientifically justified. However, any reference to specific benefits needs to be determined at the strain level. A key regulatory implication of this concept is that, even in the absence of approved strain-specific claims, use of the term ‘probiotic’ is not misleading on products formulated with a minimum level (say, 109 cfu/serving or dose) of common, well-studied probiotic species.
Are the definition and guidelines proposed in the FAO/WHO’s 2001 and 2002 documents still valid?
The probiotic definition from the 2001 FAO/WHO consultation was not grammatically correct. The basic definition is still valid, but Hill et al. (2014) updated that definition to be grammatically correct, and I support use of the updated definition. The guidelines published in the 2002 FAO/WHO document are still relevant. They call for strain identification, strain characterization, safety assessment, substantiation of efficacy in controlled trials and proper product labelling, including identification of genus, species and strain of all probiotic strains in the product and minimum level of viable probiotic delivered through the end of the product’s shelf life (I discuss this in greater depth here). However, some of the methods and suggested approaches to strain characterization in this guideline are dated.
But with regard to the concept of shared mechanisms that lead to shared benefits at taxonomic levels higher than strain, the guidelines are still relevant. It may be that benefits are shared among many strains of the same species, but probiotics still need to be identified, characterized and assessed at the strain level. This is essential for good manufacturing practices, quality control and safety.
What is the appropriate level of evidence needed to claim a health benefit for probiotics?
To me, the best approach is balancing the level of available evidence with the value of making information available to consumers and healthcare providers. One human trial might be a reasonable minimum of evidence required, but we will never have the perfect clinical trial. However, methodology exists to assess trial quality and, even in the case of limited evidence, recommendations or claims could be made as long as the limitations to the evidence are noted. I think experts should review the quality of available evidence in its totality and decide if there is a reasonable expectation (not a 100% guarantee) that the product will deliver the stated benefit. If so, I think that claims consistent with the quality of evidence should be allowed. Then it is possible for consumers and healthcare providers to make fully informed decisions.
Interestingly, healthcare providers are very comfortable recommending diets high in fiber even though evidence is lacking on the benefits of many specific dietary fibers. The evidence for specific probiotics for some health benefits is much more robust than such recommendations.
Nowadays some healthcare professionals and consumers/patients are not convinced that probiotics have enough solid scientific evidence to be used in clinical practice. What specific information should we look for on the label of a probiotic product in order to be sure that it is an effective product?
That is a shame, as there are many products with robust evidence. Top quality Cochrane systematic reviews and meta-analyses—including large numbers of studies and subjects—demonstrate this robust evidence base. [In fact, the concept of shared mechanisms provides a solid rationale for why meta-analyses of different probiotic strains can be legitimately conducted (Glanville et al. 2015).]
Unfortunately, current product labels on foods or supplement-type products will likely not tell healthcare professionals or consumers enough to determine if the product can be expected to be effective for a given health benefit. Instead, consumers and healthcare providers should turn to evidence-based guidelines and recommendations issued by medical organizations or independent reviewers (see here for a recent excellent review on clinical approaches to probiotic use written for a Pharmacy Times continuing education activity, “The Expanding Health Benefits of Prebiotics and Probiotics”. You can access the review for free but you must register to log in).
Healthcare providers are interested in helping their patients/clients manage health concerns. To the extent that a probiotic intervention has evidence of some likely benefit AND the risk of harm is quite low, I think it is reasonable to try or recommend it.
References:
Hill C, Guarner F, Reid G, et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014; 11(8):506-14. doi: 10.1038/nrgastro.2014.66.
Sanders ME, Benson A, Lebeer S, et al. Shared mechanisms among probiotic taxa: implications for general probiotic claims. Curr Opin Biotechnol. 2018; 49:207-16. doi: 10.1016/j.copbio.2017.09.007.
Hill C, Sanders ME. Rethinking “probiotics”. Gut Microbes. 2013 Jul-Aug;4(4):269-70. doi: 10.4161/gmic.25143. PMID: 23778363
Glanville J, King S, Guarner F, Hill C, Sanders ME. A review of the systematic review process and its applicability for use in evaluating evidence for health claims on probiotic foods in the European Union. Nutr J. 2015 Feb 8;14:16. doi: 10.1186/s12937-015-0004-5.