Previous preclinical and clinical data have reported that alterations in the bidirectional interactions of the central nervous system with the gut (called the gut-brain axis) may be involved in irritable bowel syndrome (IBS) pathophysiology. Interestingly, IBS symptom severity has also been related to faecal microbiota signature. However, it is still uncertain to what extent gut microbial composition can be used to classify IBS patients and to identify subgroups of patients with different clinical and behavioural phenotypes.
A new study, led by Dr. Emeran A. Mayer from the Division of Digestive Diseases at David Geffen School at University of California (UCLA) in Los Angeles (USA), has found that gut microbial composition may be associated with brain structural alterations in people with IBS.
The researchers collected behavioural measures (by using several questionnaires: The Hospital Anxiety and Depression Scale, The Patient Health Questionnaire-15, The Early Traumatic Inventory-Self Report, The Catastrophizing Subscale from the Coping Strategies Questionnaire, the Perceived Stress Scale, and a 1-month qualitative Food Frequency Questionnaire), stool samples, and structural brain images from 29 adult IBS patients (22 females) and 23 healthy control subjects (HCs) (14 females). IBS diagnosis was confirmed by Rome III symptom criteria: 11 subjects had IBS-constipation, 10 subjects had IBS-diarrhoea, and 8 subjects had either alternating (n=1), mixed (n=5), or unspecified (n=2) bowel habits.
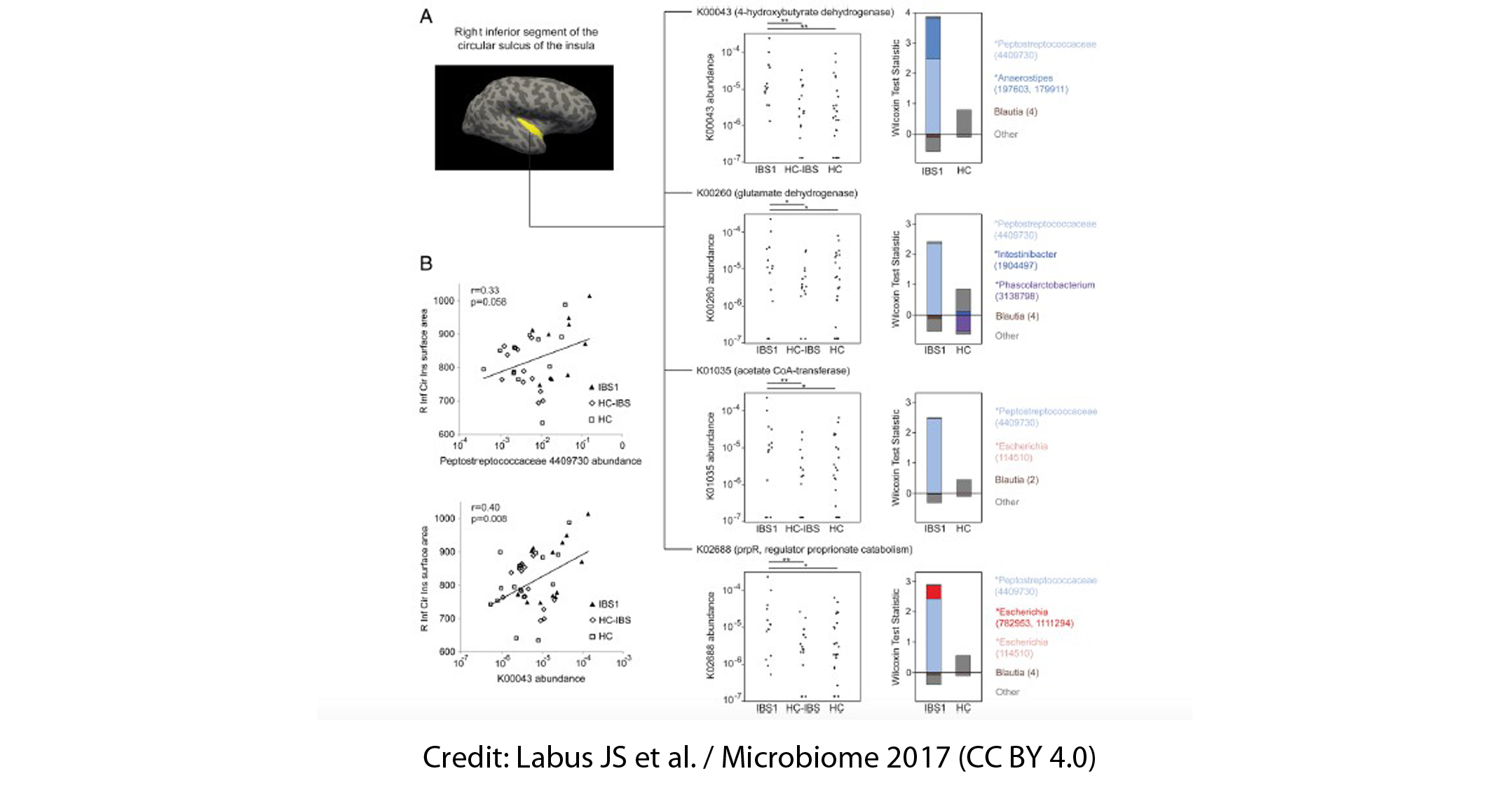
16S ribosomal ribonucleic acid (rRNA) gene sequencing data revealed two distinct subgroups within the IBS population: a group called IBS1 (n = 13), comprised of those with IBS who had a microbial profile distinct from HCs, and a group called HC-like IBS (n = 16), consisting of those with IBS who had a microbial profile indistinguishable from HCs; the third group consisted of the HCs themselves (n = 23). Members of the genera Blautia, Streptococcus, Faecalibacterium, and Bacteroides contributed to the differentiation between IBS1 and HC gut communities. Besides this, those in the IBS1 subgroup showed a history of early life trauma and longer duration of IBS symptoms. However, self-reported bowel habits, anxiety, depression, or medication use did not allow a distinction between IBS subgroups.
Gut microbial composition also correlated with the volume of brain regions involved in the processing of sensory information from the body, including structural measures of sensory- and salience-related regions. For example, the Firmicutes-associated Clostridia (higher in the IBS1 subgroup) and the Bacteroidetes-associated Bacteroidia (lower in the IBS1 subgroup) showed associations with the volume of several subcortical brain regions involved in sensory integration and modulation, and in part of the motor cortex.
Additionally, the researchers gained new insights into the relationship between early life trauma, brain development and the composition of the gut microbiome. This study showed that a history of childhood traumatic and adverse life events that occurred before the age of 18-assessed by The Early Traumatic Inventory-Self Report-was associated with structural and functional brain changes along with altered gut microbial composition.
In conclusion, for the first time researchers have identified gut microbial composition alterations correlated with brain structural alterations. The findings also suggest the possibility of a new classification of IBS patients based on gut microbial signatures rather than on clinical characteristics. Replication of this findings and mechanistic studies in experimental models are needed in order to explore the role of gut microbial communities and their related metabolomics profiles on brain signatures.
Reference:
Labus JS, Hollister EB, Jacobs J, et al. Differences in gut microbial composition correlate with regional brain volumes in irritable bowel syndrome. Microbiome. 2017; 5(1):49. doi: 10.1186/s40168-017-0260-z.